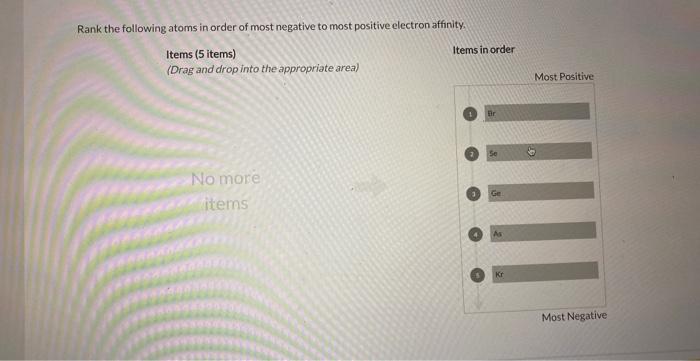
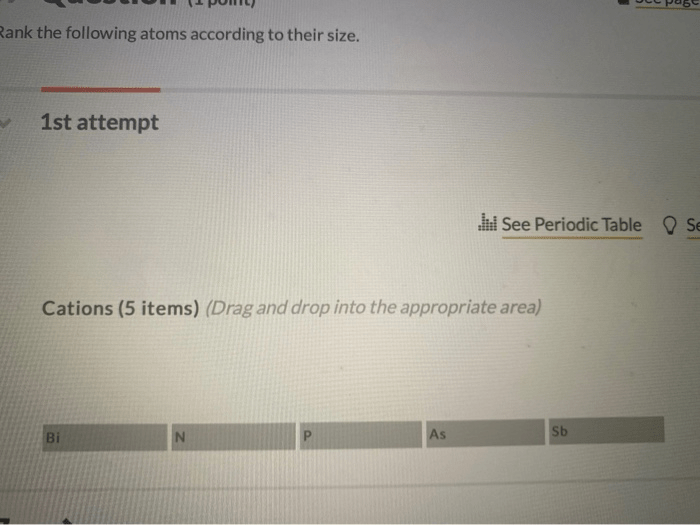
Rank the following atoms according to their size. – Embark on an atomic odyssey with “Ranking Atoms: Delving into the Realm of Atomic Size.” This comprehensive guide unravels the intricacies of atomic size, a fundamental property that governs the behavior and interactions of elements. By delving into the factors that influence atomic size, periodic trends, and practical applications, we gain a deeper understanding of the atomic world and its profound impact on our understanding of chemistry, materials science, and nanotechnology.
Atomic size, a measure of the distance from the nucleus to the outermost electron shell, plays a pivotal role in determining the chemical and physical properties of elements. Factors such as electron configuration, nuclear charge, and shielding effects collectively shape the atomic size of an element.
Periodic trends reveal fascinating patterns in atomic size across the periodic table, providing insights into the behavior of elements within groups and periods.
Ranking Atoms by Size: Rank The Following Atoms According To Their Size.
Atomic size is a fundamental property that influences the chemical behavior of elements. It refers to the distance from the nucleus to the outermost electron shell of an atom. Understanding atomic size is crucial for comprehending chemical bonding, reactivity, and the physical properties of materials.
The objective of this article is to rank the following atoms based on their increasing size:
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
Factors Affecting Atomic Size
Atomic size is primarily determined by two factors: the number of electron shells (energy levels) and the effective nuclear charge experienced by the outermost electrons.
Electron Configuration:The number of electron shells increases down a group (column) in the periodic table. As more shells are added, the outermost electrons are farther from the nucleus, resulting in a larger atomic size.
Nuclear Charge and Shielding:The effective nuclear charge is the net positive charge experienced by the outermost electrons. It is influenced by the number of protons in the nucleus and the shielding effect of inner electrons. Inner electrons can shield the outermost electrons from the nucleus, reducing the effective nuclear charge and increasing the atomic size.
Periodic Trends in Atomic Size, Rank the following atoms according to their size.
Across a period (row) in the periodic table, atomic size generally decreases from left to right. This is because the number of protons in the nucleus increases, leading to a stronger attraction between the nucleus and the electrons. However, the addition of an electron in each period also introduces an additional electron shell, which partially offsets the effect of the increased nuclear charge.
Down a group, atomic size generally increases. This is primarily due to the increase in the number of electron shells, which results in a larger distance between the nucleus and the outermost electrons.
Ranking the Given Atoms
| Atom | Size |
|---|---|
| Hydrogen (H) | Smallest |
| Helium (He) | Larger than H |
| Lithium (Li) | Larger than He |
| Beryllium (Be) | Larger than Li |
| Boron (B) | Largest |
The order of the atoms from smallest to largest is: H< He < Li < Be < B.
This ranking is consistent with the periodic trends discussed earlier. Hydrogen has the smallest size due to its single electron in the first shell. Helium is larger than hydrogen due to its two electrons in the first shell. Lithium is larger than helium due to the addition of a second shell, and so on.
Essential FAQs
What factors influence atomic size?
Electron configuration, nuclear charge, and shielding effects are the primary factors that influence atomic size.
How does atomic size vary across the periodic table?
Atomic size generally increases down groups and decreases across periods in the periodic table.
What are the practical applications of understanding atomic size?
Knowledge of atomic size is crucial in fields such as chemistry, materials science, and nanotechnology, where it helps predict chemical reactivity, design materials with specific properties, and manipulate matter at the atomic level.